TMS-007 is a member of the SMTP family and being developed as a drug treating acute ischemic stroke. About 3.3 million people die from ischemic stroke each year around the world (*1). Cerebrovascular diseases, including ischemic stroke, are serious conditions known to be the leading cause of disabilities requiring care in daily living (*2). Tissue-type plasminogen activator (t-PA; alteplase), is essentially the only available option for the thrombolytic therapy of acute ischemic stroke. Because t-PA should be administered within 4.5 hours after the onset, only 5% to 10% of patients with ischemic stroke are said to receive t-PA even in developed countries. TMS-007 is expected to treat more patients by its significantly longer time window for administration.
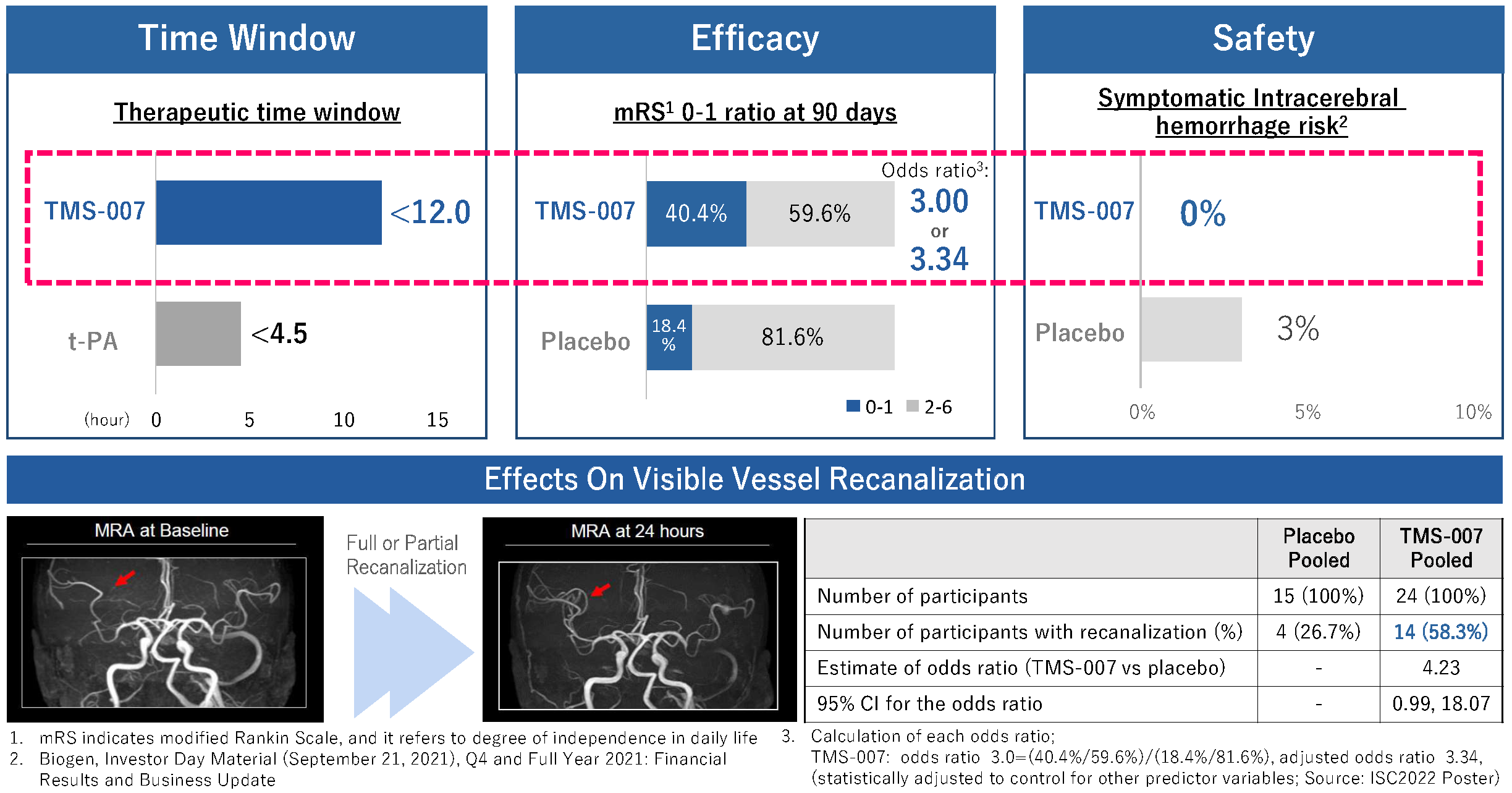
A phase 2a clinical study (*3) was planned in a randomized, placebo-controlled, double-blind design for evaluating the safety and efficacy of TMS-007 in patients with acute ischemic stroke up to 12 hours from the onset. This study achieved the primary endpoints for both safety and efficacy as summarized below (click here for a release of study results). TMS-007 can be a potential next-generation thrombolytic drug for its favorable benefit-risk balance.
TMS-007 is one of the SMTP compound with (1) prothrombolytic, (2) anti-inflammatory, and (3) antioxidative activities (click here for the pharmacological action of TMS-007).
While ischemic stroke is associated with a large number of deaths and is a serious condition that primarily results in disability requiring constant care, its therapeutic options are limited. We strive to deliver TMS-007 as a new drug to patients in the shortest possible time.
This was a multicenter, single-dose, double-blind, randomized, placebo-controlled, dose-escalation study conducted in Japan under the sponsorship of TMS Co. The study enrolled patients with acute ischemic stroke ineligible for the therapies with tissue-type plasminogen activator (t-PA) or thrombectomy. TMS-007 (at a dose of 1, 3, or 6 mg/kg) and placebo were administered within 12 hours of the symptom onset to 52 and 38 patients, respectively.