TMS-008, a member of the SMTP family compounds, exhibits anti-inflammatory and antioxidative activities but not prothrombolytic activity.
TMS-008 is effective in various animal models of inflammatory diseases. Our development is ongoing with the focus placed on acute kidney injury (AKI) and cancer cachexia. We are currently conducting GLP safety study and CMC-related development in preparation for a phase 1 clinical study.
*1 GLP (Good Laboratory Practice): The standards for appropriate conduct of non-clinical safety studies as provided by the Ministry of Health and Welfare Ordinance.
*2 CMC (Chemistry, Manufacturing and Control): “Chemistry, manufacturing, and quality controls” for pharmaceutical drug substances (active substances) and drug products (final products)
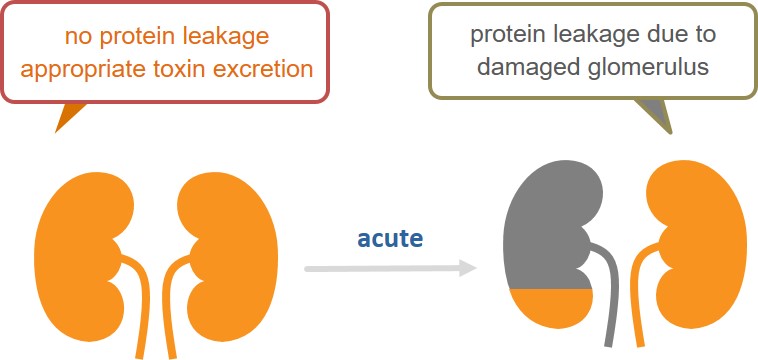
Acute kidney injury (AKI) is a condition where the kidney function rapidly deteriorates in a few hours to a few days. While accurate epidemiological information is unavailable for AKI, about 560,000 new patients have been reported each year in the US (*1). The mortality rate among hospitalized patients with AKI is 20% to 25% (*2), and frequent progression to chronic kidney disease (CKD) has been reported in those who survived. Despite these serious consequences, no medication has been approved for AKI and there are significant unmet medical needs for effective treatment in this area. AKI may result from various causes, including cardiac surgeries and adverse drug reactions.
*1 Clin J Am Soc Nephrol 1: 43–51, 2006
*2 Nephron. 2017 ; 137(4): 297–301
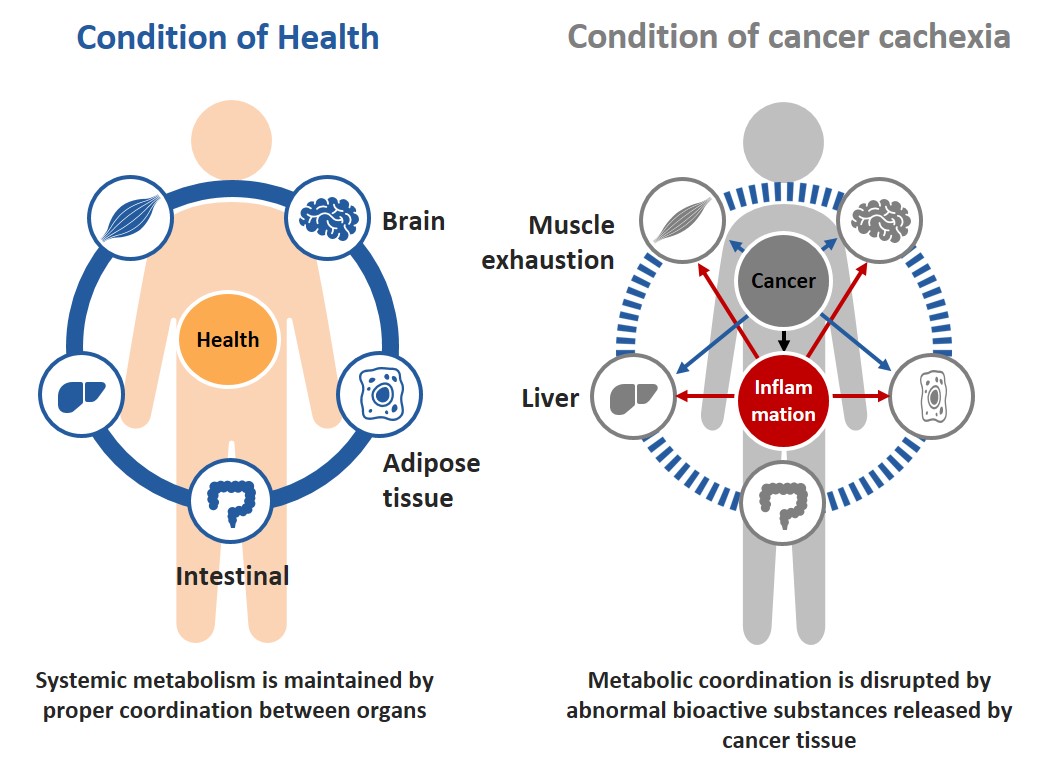
Cancer cachexia is defined as a multifactorial syndrome characterized by ongoing loss of skeletal muscle mass (with or without loss of fat mass) that cannot be fully reversed by nutritional support and leads to progressive functional impairments (*1). Cancer cachexia has been reported to affect approximately 80% of patients with advanced cancers (*1) and is considered to stem primarily from systemic inflammation.
Patients with cancer cachexia require proactive intervention because the cachectic condition often makes patients too week to withstand common cancer therapies and affects the outcome of cancer therapies.
Nevertheless, there is no approved drug that works primarily by suppressing systemic inflammation, and again, there are significant unmet medical needs for new therapies.
*1 J Jpn Soc Parenter Enteral Nutr Vol.23 No.4, 2008: 607-611
*2 Fearon K, et al. Lancet Oncol. 2011; 12(5): 489-495